Determination of Chemical Formula and the Percentage Yield of Product
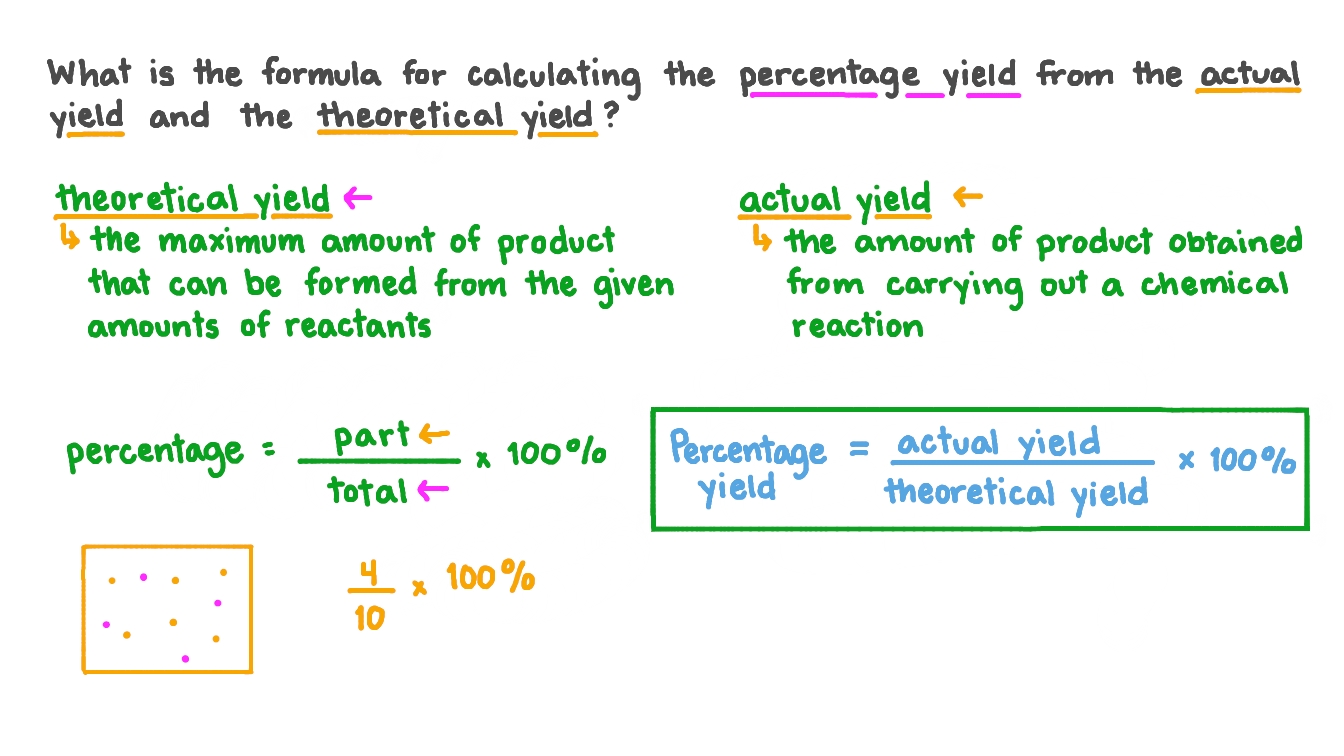
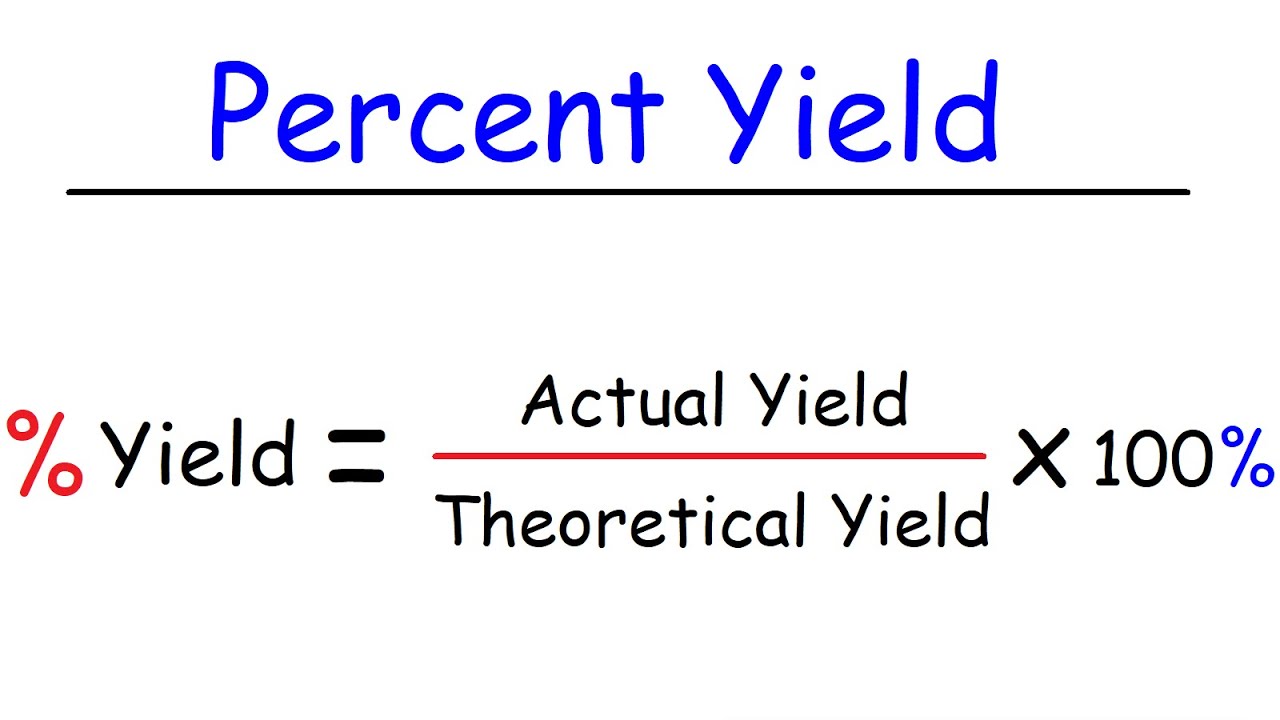
The formula for the percent yield is equal to. Percentage yield 09 18 x.

How To Calculate Theoretical Yield And Percent Yield Youtube
The experimental yield percentage is different from the theoretical percentage is because there is loss of product often occurring during the isolation and purification.
. Percentage yield beginarraylfracActual. Identify the actualexperimental yield for the given chemical reaction. Mass percentage of NH.
Mass before reaction Mass after reaction Net mass reacted Zinc Zn Iodine I 0 gram Total mass of zinc. From the ratio of the actual yield to the theoretical yield we can calculate the percentage yield. The formula is Actual Yield Theoretical Yield 100.
What is the Formula. The molecular formula of compounds can be determined from their empirical formula and either the molar mass or molecular weight. For example the chemical compound with the empirical.
We get the mole ratio from chemical analysis and from that the formula of the compound. Substitute the values in the corresponding percentage yield formula Percentage yield Actual Yield Theoretical Yield x 100. NAME EXERCISE 20 DATE LAS SECTION Report Determination of the probable Formula and the Percent Yield of the Synthesis Average mole ratio of NH to NP.
Also the hypothesis was if you had the necessary components such as the theoretical and actual yield the percent yield could be found. In this experiment we will use these principles to find the formula of the compound. The amount of product actually made compared with the maximum calculated yield is called the Percent yield.
Determination of Chemical Formula and the Percentage. Chemical Engineering Report Determination of the Probable Formula and the Percent Yield of the Synthesis Average mole ratio of NH3 to Ni2. View Experiment 11 student versionpdf from CHEMISTRY PRK1026 at University of Malaysia Sarawak.
Plug the yields from Step. In chemistry yield also referred to as reaction yield is a measure of the quantity of moles of a product formed in relation to the reactant consumed obtained in a chemical reaction usually. Mass percentage of NH in compound.
Determine these quantities in the course of the experiment. Identify the theoretical yield for the given chemical reaction.

Calculate The Theoretical Yield To Determine The Yield In A Chemical Reaction Youtube

Question Video Determining The Equation For Calculating The Percentage Yield Nagwa
How To Calculate Theoretical Yield And Percent Yield Youtube
How To Calculate Theoretical Yield And Percent Yield Youtube
No comments for "Determination of Chemical Formula and the Percentage Yield of Product"
Post a Comment